Welcome International Supporters of Homeopathy
To submit a comment to the FDA in support of homeopathy, follow the instructions on this page. Submitting a comment will take only a few minutes if you copy the comment text provided in the box below. To learn more about the situation we are facing in the United States, click here. Otherwise, continue reading to make your comment.
Are you ready to submit your comment?
1. Copy and paste the message below. The text will auto-copy to your clipboard when you click the box below.
I commend the U.S. Food and Drug Administration for carefully considering the concerns of the homeopathy community and providing adequate time to provide comments and other input which reflect those concerns.
However, I remain troubled by the Draft Guidance on homeopathy released in October 2019 by the FDA. I therefore write to support the Citizen Petition submitted by Americans for Homeopathy Choice, the country’s leading consumer homeopathy organization. The Petition is endorsed by 20 major homeopathy organizations across the United States.
The agency’s Draft Guidance would create an enforcement regime that fails to distinguish between homeopathic medicines-which when properly manufactured and labeled are nontoxic and inherently safe-and improperly labeled products that do not meet homeopathic standards.
If adopted, this guidance could lead to the removal of many popular homeopathic medicines from the market. In the process, the FDA would be abandoning decades of practice and policy that treat homeopathic medicines as unique drugs requiring a unique approach to regulation. It is ironic that the FDA’s actions would reduce consumer access to homeopathy-the safest category of drugs it regulates-and thereby force consumers to turn to pharmaceuticals which have significantly higher risks.
The Citizen Petition submitted under the federal Administrative Procedure Act by Americans for Homeopathy Choice requests that the FDA regulate homeopathy using the clear guidelines outlined in the Petition for protecting consumers from improperly manufactured and/or labeled products.
The Petition also outlines methods for assuring continuing access to the full range of nontoxic, affordable and safe homeopathic medicines on the market today. In addition, it seeks clear regulations for manufacturers that tell them what they should do in order to adhere to the law and meet the FDA’s expectations for quality and purity. Right now, the FDA’s proposed guidance provides no clear guidelines and so manufacturers are in the dark about the FDA’s expectations.
Finally, the Petition provides a framework for moving genuine homeopathic medicines into the Homeopathic Pharmacopoeia of the United States in a way that protects consumers and allows the expansion of homeopathic medicines available in the United States.
I urge the FDA to adopt the rule proposed in the Petition which would put homeopathy on a course that will ensure its safety and its continued availability to Americans who choose to make it part of their health care.
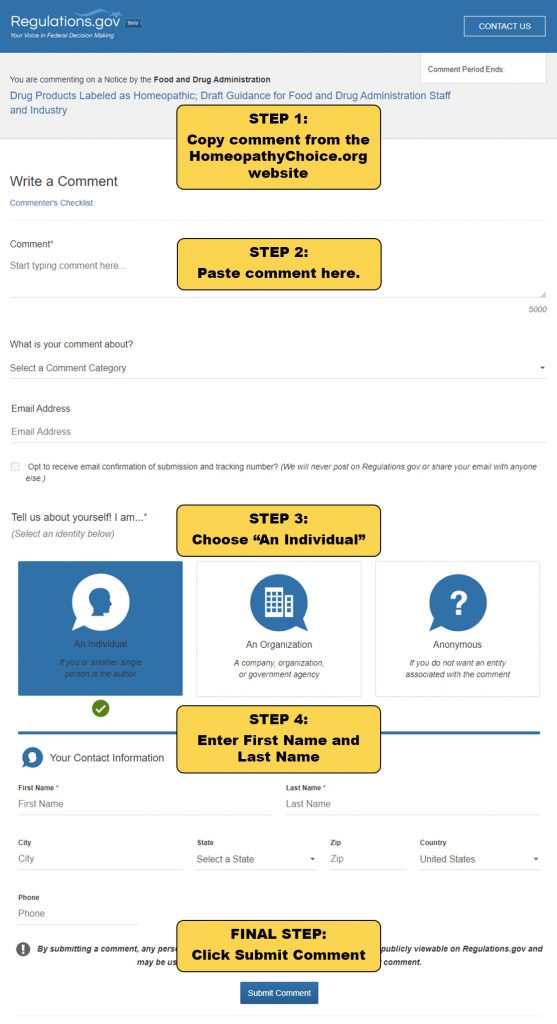
2. Click the button below and follow the instructions on the image to submit your comment.
Learn More
All of us need to work together worldwide to defend homeopathy. Here in the United States new rules proposed by the U.S. Food and Drug Administration (FDA) threaten the very existence of homeopathy.
If we do not stop those rules, the FDA could start withdrawing homeopathic remedies from the market in the second half of 2020 beginning with popular ones such as Belladonna and Nux vomica. And, what the FDA does will be noticed by drug regulators around the world.
If you ask us for the logic behind these rules, we are at a loss. There is none so far as we can tell. But we are certain that if they are allowed to go into effect, those rules will begin a countdown to zero homeopathic remedies in America. To learn more, click below.
